Chemistry is full of fascinating equations that describe how substances interact, transform, and form new compounds. One such reaction involves formic acid (HCOOH), methane or methylene group (CH₂), and water (H₂O). While at first glance, the expression HCOOH + CH₂ → H₂O may look simple, it represents an important concept in organic chemistry and environmental science. This article breaks down the reaction, explores its meaning, and highlights where it matters in practical applications.
What Does HCOOH + CH₂ → H₂O Represent?
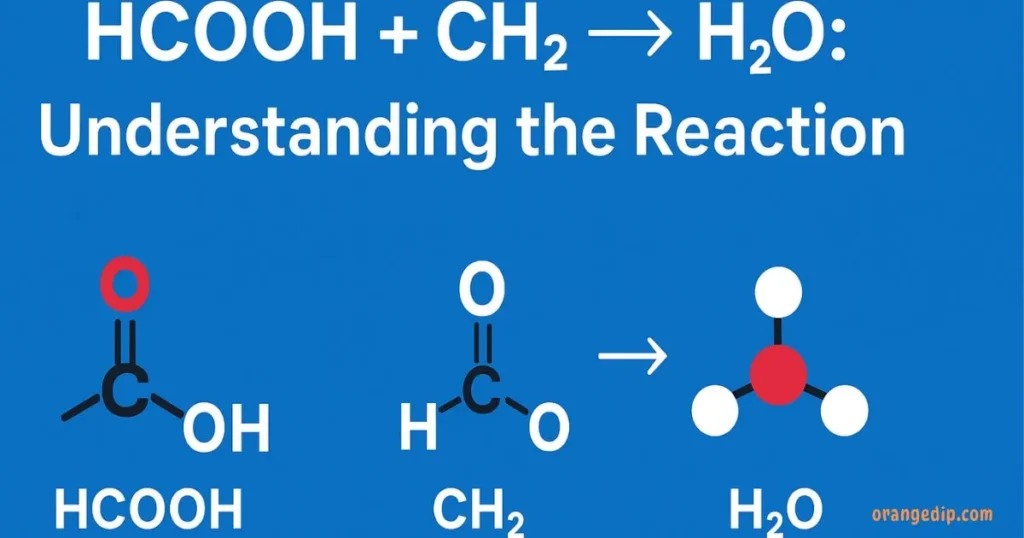
The equation HCOOH (formic acid) + CH₂ → H₂O (water) symbolizes a chemical reaction involving formic acid and a hydrocarbon group (CH₂).
- HCOOH (Formic Acid): The simplest carboxylic acid, often found in ant venom, stinging nettles, and used industrially.
- CH₂ Group: Represents a methylene unit, common in hydrocarbons and organic reactions.
- H₂O (Water): The most stable and universal byproduct of countless chemical reactions.
This reaction highlights oxidation-reduction processes in which electrons are transferred, resulting in the release of water. In simplified terms, formic acid acts as a reducing agent while undergoing decomposition or combination with hydrocarbons.
Breaking Down the Reaction Step by Step
To understand it more clearly, let’s analyze what happens in stages:
- Reactants Interaction
- Formic acid interacts with a methylene group or related hydrocarbon fragment.
- Bond Rearrangement
- Hydrogen atoms shift, bonds break and reform, leading to the release of water.
- Products Formation
- The main outcome is H₂O, though depending on conditions, additional organic compounds may form.
This process mirrors organic combustion reactions, where hydrocarbons combine with oxygen to produce carbon dioxide and water.
Why Is the Reaction Important?
This reaction is more than a textbook example—it has several implications in organic chemistry, industrial synthesis, and environmental processes.
- Organic Synthesis – Used in laboratories for creating more complex molecules.
- Environmental Chemistry – Explains how natural compounds like formic acid break down in ecosystems.
- Industrial Uses – Applied in fuel technology, polymer production, and chemical manufacturing.
By understanding HCOOH + CH₂ → H₂O, chemists gain insights into reaction pathways that influence both research and practical applications.
How Does Formic Acid Behave in Reactions?
Formic acid is unique because it exhibits properties of both carboxylic acids and aldehydes. This dual nature makes it highly reactive in certain reactions.
- As a reducing agent, it donates hydrogen.
- As an acid, it releases protons (H⁺) easily.
- Under heat, it decomposes into carbon monoxide and water.
This reactivity explains why formic acid is so often studied in relation to water-producing reactions.
Read More: ScoopUpdates.com: Your Ultimate Source for News, Trends, and Insights
What Are the Real-Life Applications of This Reaction?
The breakdown of formic acid with hydrocarbons or under heat conditions provides insights into:
- Fuel Cells: Formic acid is tested as a hydrogen source for direct fuel cells, where hydrogen reacts with oxygen to generate electricity and water.
- Industrial Solvents: The byproducts of such reactions are used in leather, textile, and rubber processing.
- Environmental Studies: Scientists study these reactions to monitor pollution breakdown in soil and water.
- Pharmaceutical Chemistry: Helps in synthesizing active pharmaceutical ingredients (APIs).
Frequently Asked Questions (FAQs)
1. What is the chemical formula HCOOH?
HCOOH is formic acid, the simplest carboxylic acid. It occurs naturally in ant venom and is widely used in industry for tanning leather, producing textiles, and as a preservative.
2. Why is water (H₂O) a common product in chemical reactions?
Water is highly stable, making it a natural byproduct when hydrogen and oxygen atoms rearrange during chemical processes like combustion, condensation, and neutralization.
3. How is formic acid used in green energy?
Formic acid is considered a promising hydrogen storage material for fuel cells. It can release hydrogen and water under controlled reactions, supporting sustainable energy solutions.
4. Is the reaction HCOOH + CH₂ → H₂O reversible?
Generally, this type of reaction is not reversible because water is very stable and its formation drives the reaction forward. However, under special catalytic conditions, reverse reactions can sometimes be engineered.
5. Where else is the CH₂ group important in chemistry?
The CH₂ (methylene) group is crucial in organic compounds like alkanes, alkenes, and polymers. It forms the backbone of hydrocarbons and plays a role in plastics, fuels, and biological molecules.
Final Thoughts
The equation HCOOH + CH₂ → H₂O is more than just a chemical expression—it represents a fundamental principle of how molecules interact to form stable products like water. From fuel cells to environmental science and organic synthesis, understanding this reaction offers valuable insights into modern chemistry.
By breaking it down, we see that such reactions are not just academic but deeply connected to green technology, industry, and everyday life.